
/GettyImages-170075160-56a133b73df78cf772685a14.jpg)
The magnitude of the wave function at a particular point in space is proportional to the amplitude of the wave at that point.If you are the captain of a ship trying to intercept an enemy submarine, you need to deliver your depth charge to the right location at the right time. For electrons, we can ignore the time dependence because we will be using standing waves, which by definition do not change with time, to describe the position of an electron.įigure 6.20 The Four Variables (Latitude, Longitude, Depth, and Time) Required to Precisely Locate an Object For example, if you wanted to intercept an enemy submarine, you would need to know its latitude, longitude, and depth, as well as the time at which it was going to be at this position ( Figure 6.20 "The Four Variables (Latitude, Longitude, Depth, and Time) Required to Precisely Locate an Object"). Three specify the position in space (as with the Cartesian coordinates x, y, and z), and one specifies the time at which the object is at the specified location.
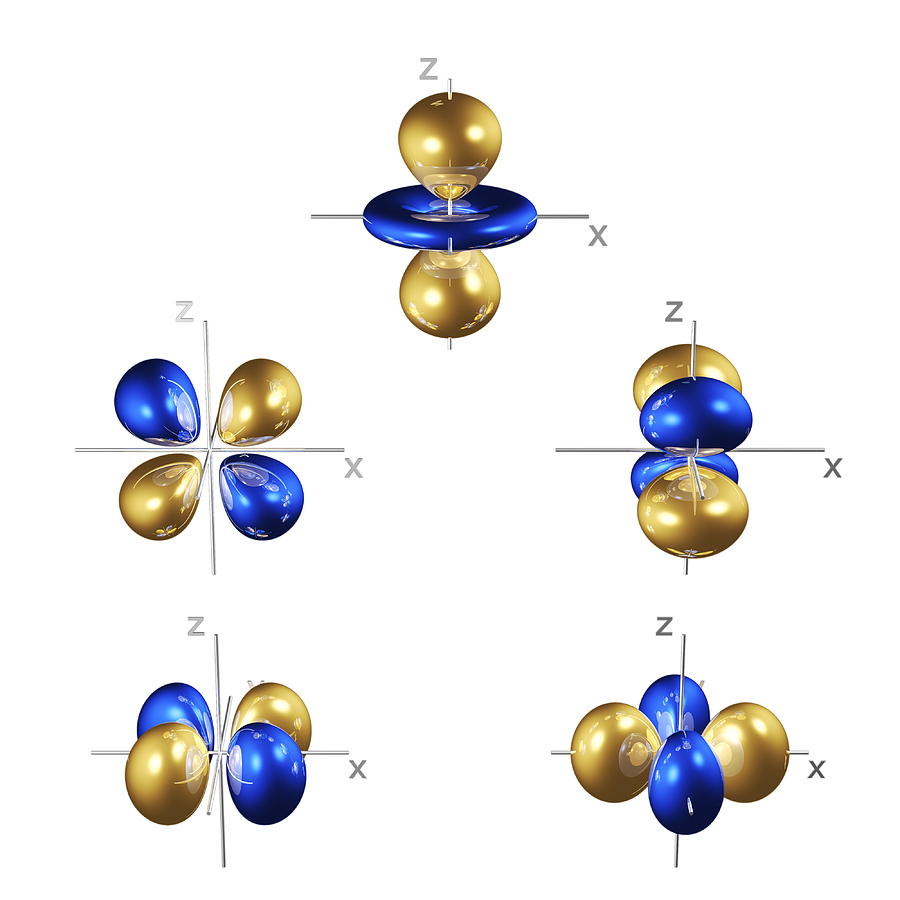
A fourth variable is usually required to fully describe the location of objects in motion. A wave function uses three variables to describe the position of an electron.The properties of wave functions derived from quantum mechanics are summarized here: Thus each wave function is associated with a particular energy E. is a mathematical function that relates the location of an electron at a given point in space (identified by x, y, and z coordinates) to the amplitude of its wave, which corresponds to its energy. A wave function (Ψ) A mathematical function that relates the location of an electron at a given point in space to the amplitude of its wave, which corresponds to its energy.


 0 kommentar(er)
0 kommentar(er)
